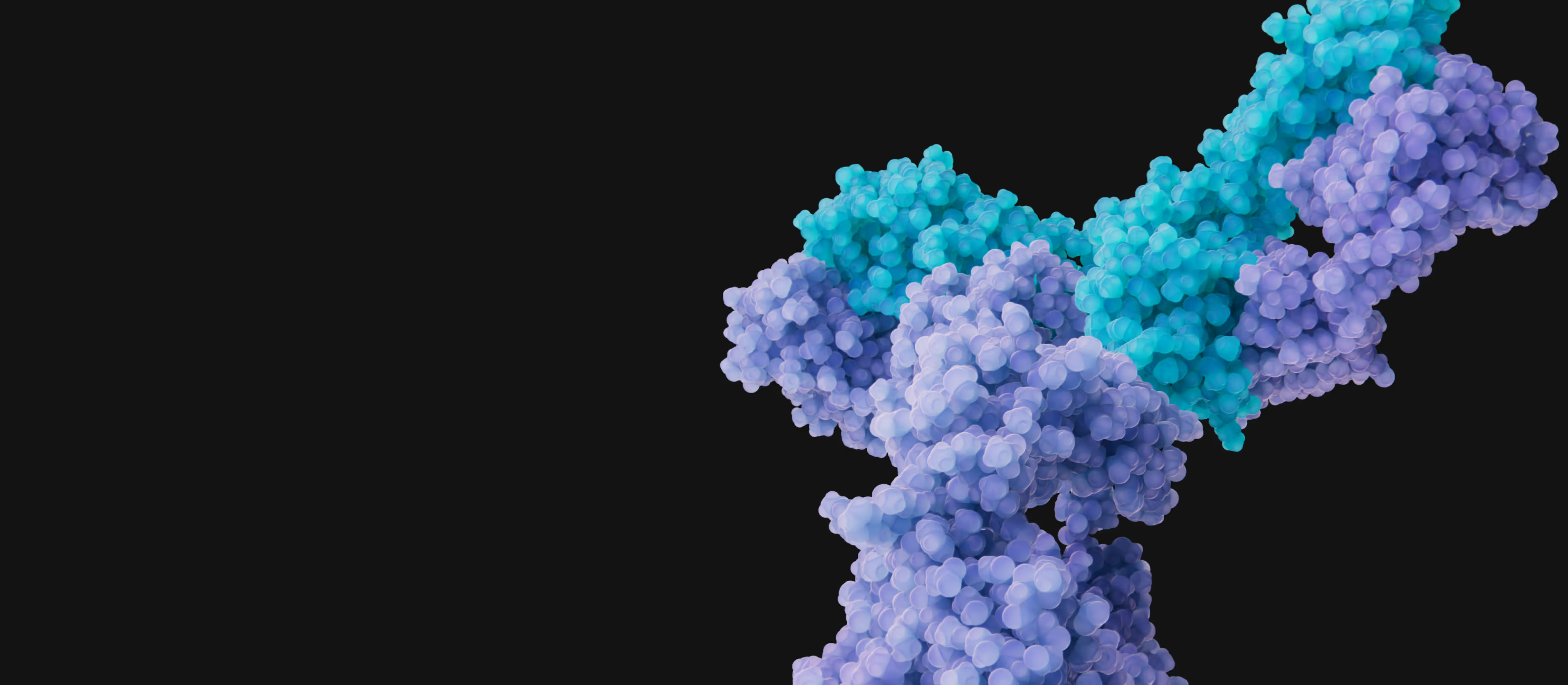
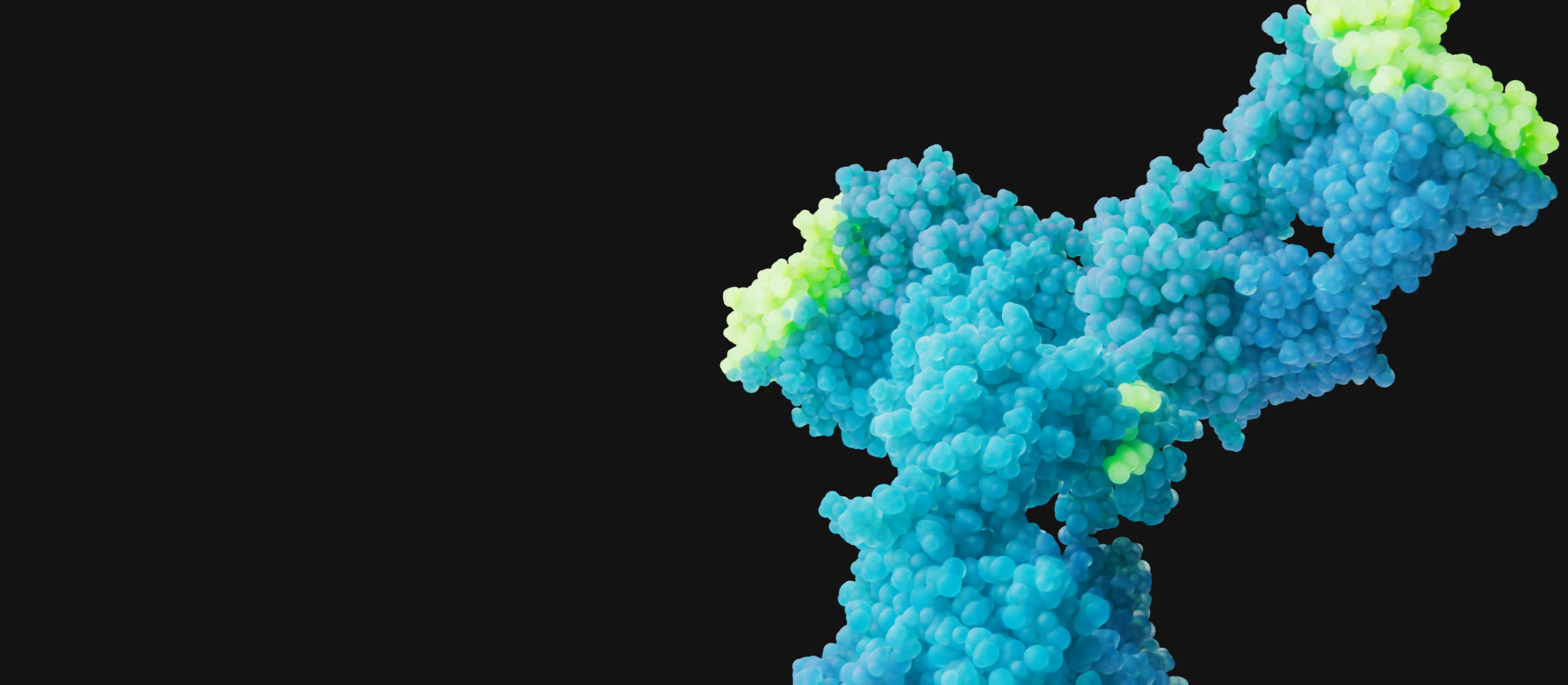
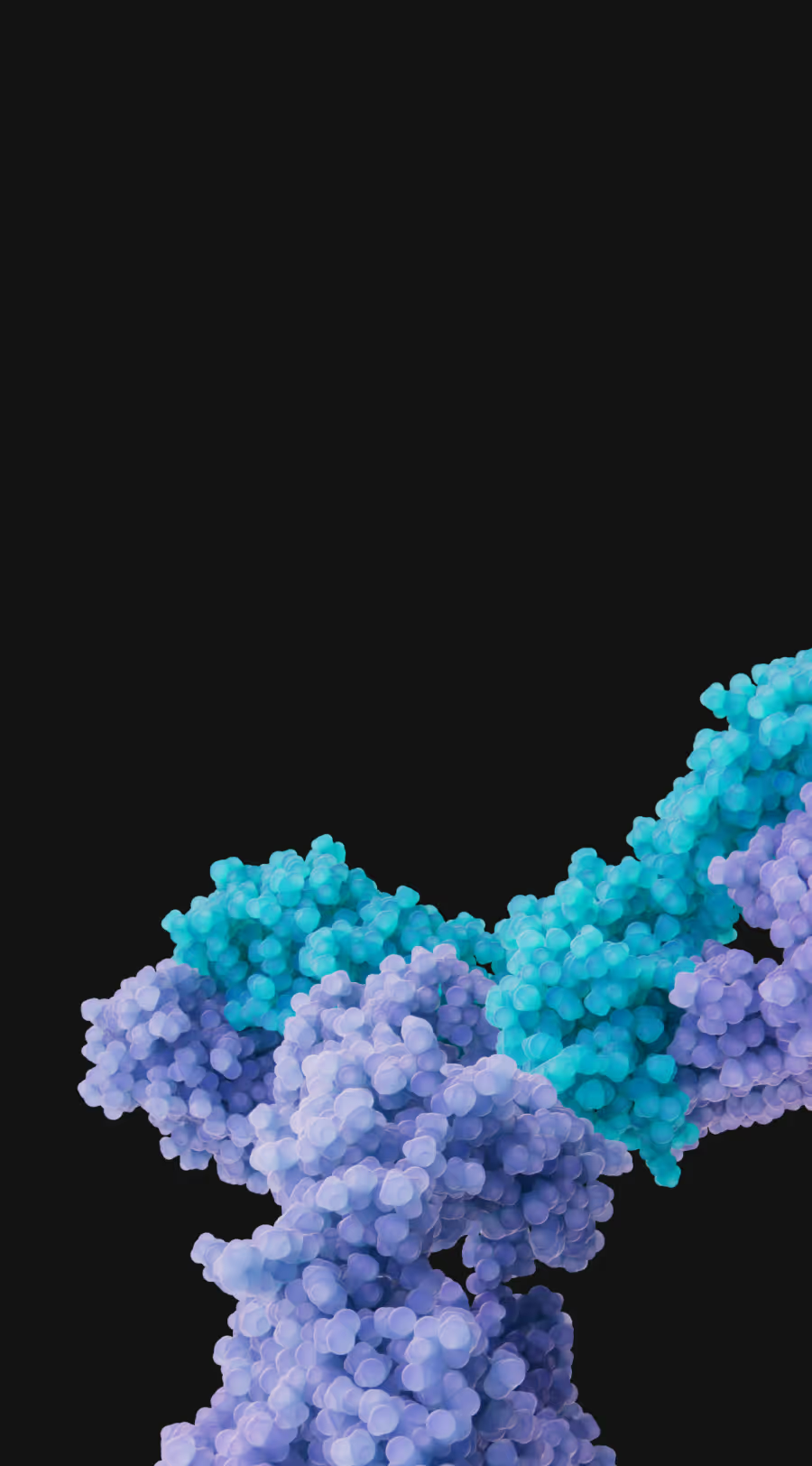
Establishing DR3 as a Foundational Target in IBD and Beyond
Shattuck is developing a potentially first-in-class DR3 blocking antibody for the treatment of Inflammatory Bowel Disease (IBD) and other inflammatory and immune-mediated diseases. Targeting immune receptors, like DR3, may provide superior efficacy than targeting immune ligands such as TL1A. In addition, DR3 blocking antibodies are not expected to cause immune complex formation and thus may have a superior immunogenicity profile in comparison to TL1A blocking antibodies.

Our Pipeline
Shattuck’s lead DR3 blocking antibody, SL-325, demonstrates superior inhibition and efficacy in preclinical assays over therapies targeting TL1A. SL-325 harnesses the power of high affinity, epitope specificity and critical Fc-silencing to block TL1A from engaging DR3 expressed on multiple immune cells that drive inflammation.
News

October 2, 2025
Shattuck Labs Announces Participation in Upcoming Wedbush Securities Key Opinion Leader (KOL) and Company Panel.

October 2, 2025
Shattuck Labs Announces Participation in Upcoming Wedbush Securities Key Opinion Leader (KOL) and Company Panel.

October 2, 2025
Shattuck Labs Announces Participation in Upcoming Wedbush Securities Key Opinion Leader (KOL) and Company Panel.
Events

October 2, 2025
Berlin, Germany
Shattuck Labs Announces Participation in Upcoming Leerink Partners Therapeutics Forum: I&I and Metabolism

October 2, 2025
Berlin, Germany
Shattuck Labs Announces Participation in Upcoming Leerink Partners Therapeutics Forum: I&I and Metabolism

October 2, 2025
Berlin, Germany
Shattuck Labs Announces Participation in Upcoming Leerink Partners Therapeutics Forum: I&I and Metabolism














